Equilibrium
& Kinetics
Notes,
Chapter 17
There are three main areas that concern us in this chapter: thermodynamics, kinetics, and equilibrium. Thermodynamics was covered in Pt. I.
I. Kinetics
a. Kinetics is the area of chemistry that is concerned with how fast reactions occur.
b. Thermodynamics is concerned with whether or not a reaction is spontaneous. That is, thermodynamics tries to answer the question, “Will it happen?” Kinetics tries to answer the question, “How fast does it happen?”
c. Now, consider the diagrams shown below for two different chemical reactions.
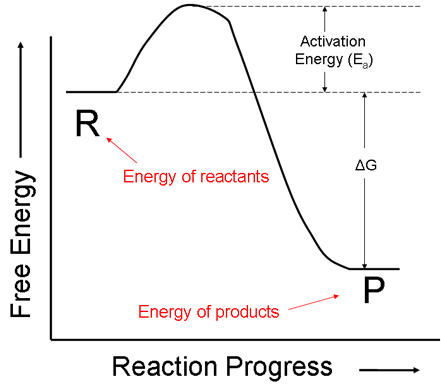
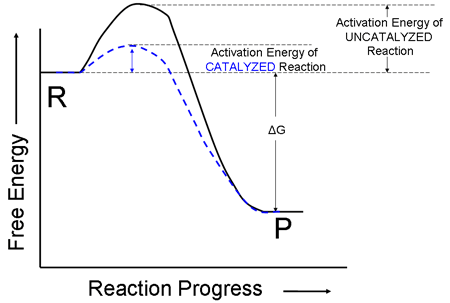
i. First, let us consider a reaction that gives off more energy than it takes in. That is, the following diagram shows a reaction for which the initial energy of the reactants is greater than the energy of the final products.
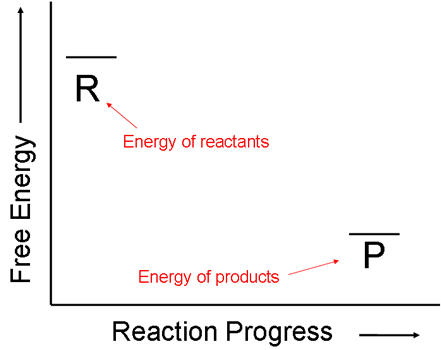
ii. Even though the net change in free energy, ΔG, of this reaction is negative, the energy of the reactants does not continuously decrease as the reaction progresses. The reactant molecules must first bash into each other in order to react. When they do, the energy of the system actually increases at first. This amount of energy that must first be supplied to the system is called the activation energy.
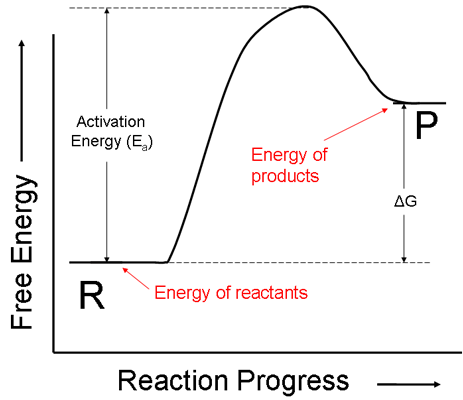
iii. The energy diagram for a reaction that increases in free energy is shown below. Notice that the ΔG for this reaction is positive (reaction is going “uphill” more than it is going downhill). Notice also that the activation energy is still the amount of energy that is required to go from the energy of the reactants to the top of the energy hill.
d. There are several ways to raise the rate of a chemical reaction.
i. Raising the temperature increases the rate of reaction
1. When the temperature of a substance is increased, the particles n that substance move faster. This means that the particles that are reacting with one another hit each other more often. The more times that two reacting particles knock into each other, the more likely it is that they will have a successful collision that leads to new products. This is means that when a substance is hot, more particles are getting to the top of the “energy hill” in the diagram above, which means more particles will “fall over” to the products side of the diagram.
ii. Increasing surface area/crushing the reaction mixture increases the rate of reaction
1. If one of the reactants is a solid, then crushing the solid into smaller pieces will increase the rate of reaction. This increase occurs because the surface area of the solid reactant that is in contact with the other reactant(s) has been increased.
2. The reaction of wood with O2 in the air, for instance, is much faster when the wood has been ground into sawdust than when the wood is in the form of a huge log.
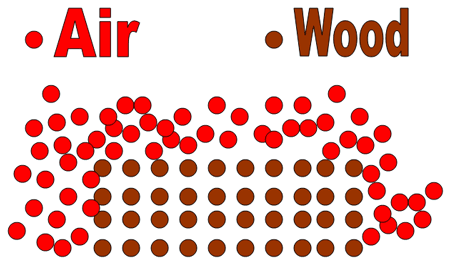
Figure 1 A log of wood, which has a relatively small surface area, reacting in air.
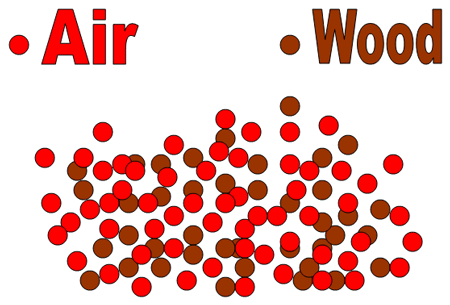
Figure 2 Wood in the form of sawdust reacting with air. The sawdust has a much greater surface area than does a log. The greater surface area allows more particles of the reactants to hit each other, resulting in a faster rate of reaction.
iii. Stirring/agitating reaction mixture.
1. Again, more particles of reactant are forced to bump into each other than if they weren’t stirred. Consider that stirring a cup of coffee w/sugar in it makes the sugar dissolve more quickly. Sugar that is only in contact with the part of the coffee that has already been saturated with sugar will not dissolve. If the mixture is stirred, more successful collisions (and reaction) will take place.
iv. Increasing concentration.
1. A log of wood will burn more quickly in pure O2 (100% oxygen) than in air (only about 21% oxygen).
v. Adding a catalyst increases the rate of reaction
1. A catalyst is a substance which increases the rate of a chemical reaction, but is not itself actually consumed or produced in the reaction. A catalyst simply positions or otherwise interacts with the reacting particles so that they react with each other more easily. Therefore, the energy needed for the particles to react in the presence of a catalyst is reduced, and more particle collisions are successful ones. The energy diagram for a catalyzed reaction will have the same starting and ending energy, and thus the size of ΔG is not changed. Also, the SIGN of ΔG does not change.
II. Equilibrium
a. Equilibrium is the exact balancing of two processes, one of which is the opposite of the other.
b. We have seen an example of equilibrium when we examined water that is in an enclosed container that has a headspace:
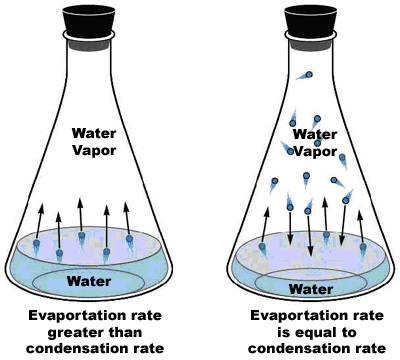
The flask on the right is at equilibrium.
The flask on the left is on the way to achieving equilibrium, but is not at equilibrium presently.
Remember, when the reaction is at equilibrium, the amounts of vapor and liquid are not necessarily equal. What is “equal”, then, about equilibrium? The answer is that at equilibrium the rate of the forward process and the rate of the reverse process are equal.
For the flask at equilibrium, we write:
H2O (l) Û H2O (g)
That arrow that you see is the closest that I can come to a double arrow in Microsoft Word. Usually, a reversible reaction is written as
![]()
c. Just as the process of evaporation/condensation is reversible, there are also numerous chemical reactions which are considered reversible.
N2O4 (g) Û 2NO2 (g)
d. Going left to right is the forward process. Going backwards (right to left) is the reverse process. Even though this reaction occurs in both the forward and reverse directions simultaneously, the left side is always called the reactants side. The right side is always called the products side.
e. The position of equilibrium is the qualitative way of describing the ratio of products to reactants when the reaction is at equilibrium. The position of equilibrium can change, or shift, in response to changing the temperature, pressure, or concentrations of the reaction mixture.
f. The ratio of reactants to products at equilibrium is described by a constant number, called Keq, pronounced “the constant of equilibrium,” or simply K.
g. The Keq is computed by the following formula for our chemical reaction:
![]()
h. Here is another example:
4NH3 (g) + 7O2 (g) Û 4NO2 (g) + 6H2O (g)

i. Writing the equilibrium constant expression for a reaction that has solids or liquids is more complex. Because pure solids and pure liquids do not have concentrations, they are not represented in the equilibrium constant expression for a reaction. However, aqueous solutions and gases do have concentrations, and therefore are included in the Keq expression.
j. Certain reactions have more than one phase of matter present once a state of equilibrium has been achieved. Such reactions are examples of heterogeneous equilibria. (Singular: heterogeneous equilibrium.) Here are some examples:
1. CaCO3 (s) Û CaO (s) + CO2 (g)
The calcium carbonate is a solid, so it does not go into the Keq expression.
![]()
2. CaO (s) + CO2 (g) Û CaCO3 (s)
The calcium carbonate is a solid, so it does not go into the Keq expression.
![]()
3. HCl (aq) + H2O (l) Û H3O+ (aq) + Cl- (aq)
Water is a liquid, so it is not represented into the Keq expression.
![]()
k. So far we have written down the equilibrium constant expressions for a number of chemical reactions. In a sense, all we have done so far is learn how to “set up” the problems. Now, we will plug in numbers for the concentrations and solve for the value of the equilibrium constant, K.
l. Remember the “qualitative” meaning of the quotient “K”:
i. If K >1, then [P] >[R], and therefore the reaction is “product-favored.”
1. This means that when this reaction comes to equilibrium, the rate of the forward reaction will indeed be equal to the rate of the reverse reaction. However, the concentrations of the products will be greater than the concentrations of the reactants once equilibrium has been achieved.
2.
Imagine if Mr.
Mac is giving a dollar bill to Donald Trump every time Donald Trump gives Mr.
Mac a dollar bill. The rate of exchange is equal, but Donald Trump is far
richer:
Reaction
between Mr. Mac’s Money and Donald Trump’s money:
Mr. Mac’s Money Û Donald
Trump’s money

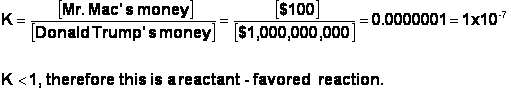
ii. If K <1, then [P] <[R], and therefore the reaction is “reactant-favored.”
1. This means that when this reaction comes to equilibrium, the rate of the forward reaction will indeed be equal to the rate of the reverse reaction. However, the concentrations of the products will be smaller than the concentrations of the reactants once equilibrium has been achieved.
2.
Again, imagine
if Mr. Mac is giving a dollar bill to Donald Trump every time Donald Trump
gives Mr. Mac a dollar bill. The rate of exchange is equal, but Donald Trump is
far richer. I will write the equation in the opposite order this time:
Reaction
between Mr. Mac’s Money and Donald Trump’s money:
Donald Trump’s money Û Mr.
Mac’s Money
Example Problem: Ammonia is produced from nitrogen and hydrogen in the following reaction:
N2 (g) + 3H2 (g) Û 2NH3 (g)
The reaction was examined once it achieved equilibrium and the following reaction concentrations were recorded:
[N2] = 4.9 x 10-4 M [H2] = 2.1 x 10-3 M [NH3] = 0.34 M
Using these concentrations, determine the value of the equilibrium constant, K, for this reaction.
Answer:
First, we must write down the equilibrium constant expression:
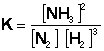
Then, plug the values into the equation:
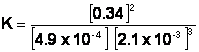
![]()
![]()
![]()
Further analysis of this answer: You may have asked yourself the following question: “Just what is the meaning of ‘K=2.5 x 1010?’” Well, this number is a measure of how much greater the concentrations of products are than the reactants, because K is equal to the concentrations of the products divided by the concentrations of reactants. The ratio is much greater than one, so [P] is much greater than [R]. Thus, this is a product-favored reaction, because K >>1.
m. Le Châtelier’s Principle
i. Named after Henri Louis Le Châtelier (1850-1936, France), this principle states that if a chemical system at equilibrium is disturbed from equilibrium, the concentration of the reactants and products will adjust so that equilibrium is re-achieved.
ii. Another simple way of saying this is if a bunch of chemicals in a reaction do not divide out to the right number, then the concentrations of those chemicals will go up or down so that they once again divide out to the correct number (K).
iii. In this course, you will be given the chemical equation for some reaction at equilibrium, you will be told what change was made to the system, and you will be asked how the system must adjust in order for the system to re-achieve equilibrium.
iv. Changes to equilibrium, and their effect on equilibrium position:
1. Changes in concentration
2. Changes in pressure
3. Changes in temperature
a. Exothermic reactions
b. Endothermic reactions
4. Changes in volume of reaction vessel
5. Addition of an inert gas
6. Addition of a catlayst
v. Example:
vi. Example:
vii. Example:
viii. Example:
ix. Summary of LeChâtelier’s Principle
2A (g) + B (liq) Û 2C (s) + 3D (aq) + E (g) ΔH° = - 55 kJ
This can also be written as a thermochemical equation:
2A (g) + B (liq) Û 2C (s) + 3D (aq) + E (g) + 55kJ
|
Change made to system |
|
Response of system to that change |
|
|
|
|
|
Increase amount/conc. of A |
→ |
Shifts right |
|
Decrease amount/conc. of A |
→ |
Shifts left |
|
Increase amount of B |
→ |
No effect |
|
Decrease amount of B |
→ |
No effect |
|
Increase amount of C |
→ |
No effect |
|
Decrease amount of C |
→ |
No effect |
|
Increase conc. of D |
→ |
Shifts left |
|
Decrease conc. of D |
→ |
Shifts right |
|
Increase amount of E |
→ |
Shifts left |
|
Decrease amount of E |
→ |
Shifts right |
|
Increase the temperature of the system |
→ |
Shifts left |
|
Decrease the temperature of the system |
→ |
Shifts right |
|
Increase the pressure of the system |
→ |
Shifts right |
|
Decrease the pressure of the system |
→ |
Shifts left |
|
Increase the volume of the reaction vessel |
→ |
Shifts left |
|
Decrease the volume of the reaction vessel |
→ |
Shifts right |
|
Add a catalyst |
→ |
No effect |
|
Add an unreactive substance that is not present in the chemical equation |
→ |
No effect |
|
“When THIS is done to the chemical reaction . . . |
|
. . . THIS is how the reaction will respond.” |